Addition of Hydrogen Halides to Alkenes Mechanism
A recent application is the generation of highly reactive aryl radicals which are useful arylating reagents in synthesis by photoinduced electron transfer PET from photoredox catalysts to suitable precursors followed by bond scission 8 9However the choice of aryl radical precursors is currently limited to electron-poor arenes such as diazonium 6 10 or. If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms the halogen is found preferentially at the carbon with fewer hydrogen.

Regioselectivity Of Hydrogen Halide Addition Markovnikov S Rule Youtube
With such capabilities they can.
. CH 3 CHCH 2 HI CH 3 CHICH 2 H. The elements of water can be added to the doublebonded carbons of an alkene in either a Markovnikovs or an antiMarkovnikovs manner. Markovnikovs Rule with Practice Problems.
Both equivalents of HX add to the triple bond in the same step. If its not in the textbook chances are it. As shown in the following figure a hydrogen ion catalyzes the Markovnikovs addition.
Matching alcohols to their names I. The carbonyl compounds in which carbon of carbonyl group is bonded to carbon or hydrogen and oxygen of hydroxyl moiety -OH are known as carboxylic acids while in compounds where carbon is attached to carbon or hydrogen and nitrogen of -NH2 moiety or to halogens are called amides. The S N 2 doesnt happen for secondary alcohols.
In addition to primary amines secondary amines led to reductive coupling with nitriles and provided tertiary chiral amines in up to 85 yields and 99 ee Fig. Mechanism The mechanism of the reaction involves the following three steps. Addition Reactions of Alkenes.
For instance alkanes alkynes or alkenes the amount of bonded hydrogen decreases in alkenes and alkynes. Represented by R-Mg-X where R is an alkyl or aryl group while X is a halogen the Grignard reagent easily forms a carbon-carbon. Reaction Mechanism Click Here for Sample Questions The haloalkanes or aryl halides with sp 3 or sp 2 hybridised carbon atoms when reacted with Magnesium metal give Grignard reagent which is an organometallic compound.
S N 1 S N 2 E1 or E2 the Largest Collection of Practice Problems. Drawing formulas from names. Formation of protonated alcohol.
The ability of hydrocarbons to bond to themselves is known as catenation. Products 214 to 215. The primary alcohols elimination reactions follow the E2 mechanism whereas the secondary and tertiary alcohols.
Only one textbook in this admittedly incomplete sample mentions the S N i mechanism at all. Which of the following options correctly describe the addition of a hydrogen halide to an alkyne. Dehydration can be performed in a 3-step mechanism.
This is mainly due to the self-bonding or catenation of carbon that prevents the complete saturation of the hydrocarbon by the formation of double or triple bonds. Theres no warning sign saying wait. Mechanism of Dehydration of Alcohols.
The NHC ligands on the cluster as revealed by density functional theory DFT calculations are key factors in determining the selectivity of the. Drawing formulas from names. Carbon and hydrogen while in the ketones it is bonded to two carbon atoms.
Acid-Catalyzed Hydration of Alkenes with Practice Problems. A vinyl halide is produced after the addition of one equivalent of HX. In four textbooks where SOCl 2 is mentioned the reaction is shown as proceeding through an S N 2 mechanism.
Formation of alkenes. Hydrohalogenation is the addition of hydrogen halides such as HCl or HI to alkenes to yield the corresponding haloalkanes. From alkenes i By acid catalysed hydration.
Drawing formulas from names. Select all that apply. Drawing alkyne formulas from names.
The Role of the Solvent in S N 1 S N 2 E1 and E2 Reactions. Drawing alkene formulas from names. In case of unsymmetrical alkenes the addition reaction takes place in accordance with Markovnikovs rule Unit 13 Class XI.
Notably for products 210 and 214 12 to 14 racemization is observed which can be explained by the comparably high acidity of the α-hydrogen atom in the case of the starting 2. Similarly groups that favor ionization of the halogen may generate a transition state with substantial positive charge on the alpha-carbon and only a small degree of CH breaking. Drawing alcohol formulas.
Delydrobalogenation of vinyi halides is essentially. Electrophilic addition to conjugated dienes occurs through 12 and 14-addition mechanism out of Q. When two equivalents of HX add to an alkyne the halogen atoms will end up on.
For example if the Rgroups on the beta-carbon enhance the acidity of that hydrogen then substantial breaking of CH may occur before the other bonds begin to be affected. S N 1 S N 2 E1 E2 How to Choose the Mechanism. Dehydration of alcohols follows the E1 or E2 mechanism.
The antiMarkovnikovs addition results from a hydroborationoxidation reaction. Alkenes react with water in the presence of acid as catalyst to form alcohols. Such an encirclement arrangement and open stereochemical framework endow the single Pd atom with the power to activate hydrogen H 2 thereby enabling the catalytic hydrogenation of alkenes under mild conditions.
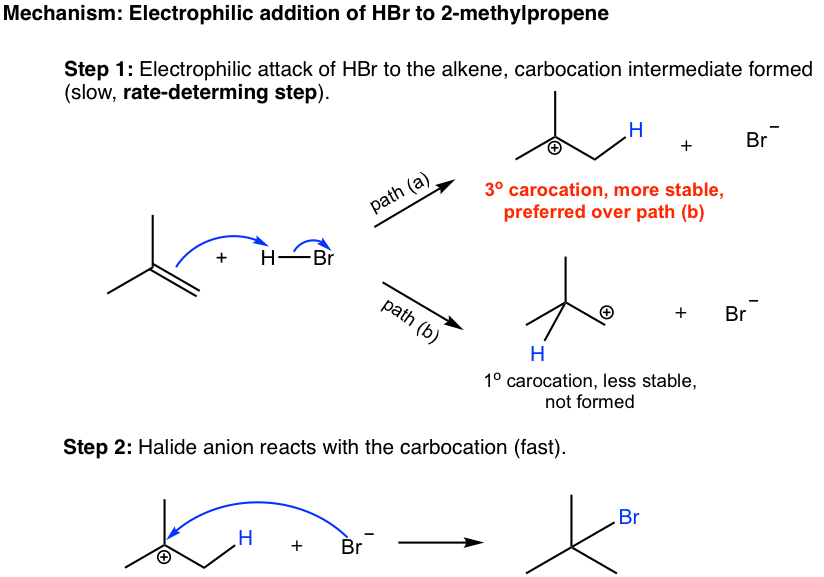
Electrophilic Addition Of Hydrogen Halides Chemistry Libretexts
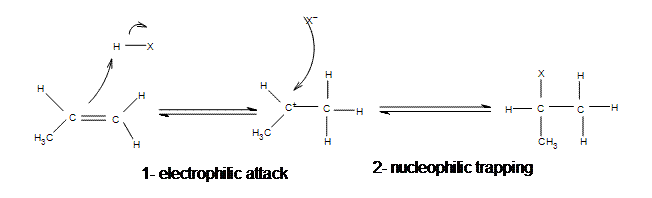
9 2 Addition Of Hydrogen Halides To Symmetrical Alkenes Chemistry Libretexts

Electrophilic Addition Reactions Of Alkenes Mcc Organic Chemistry

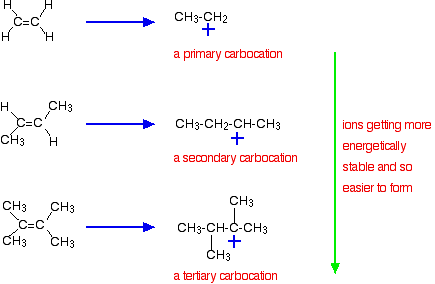
10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I
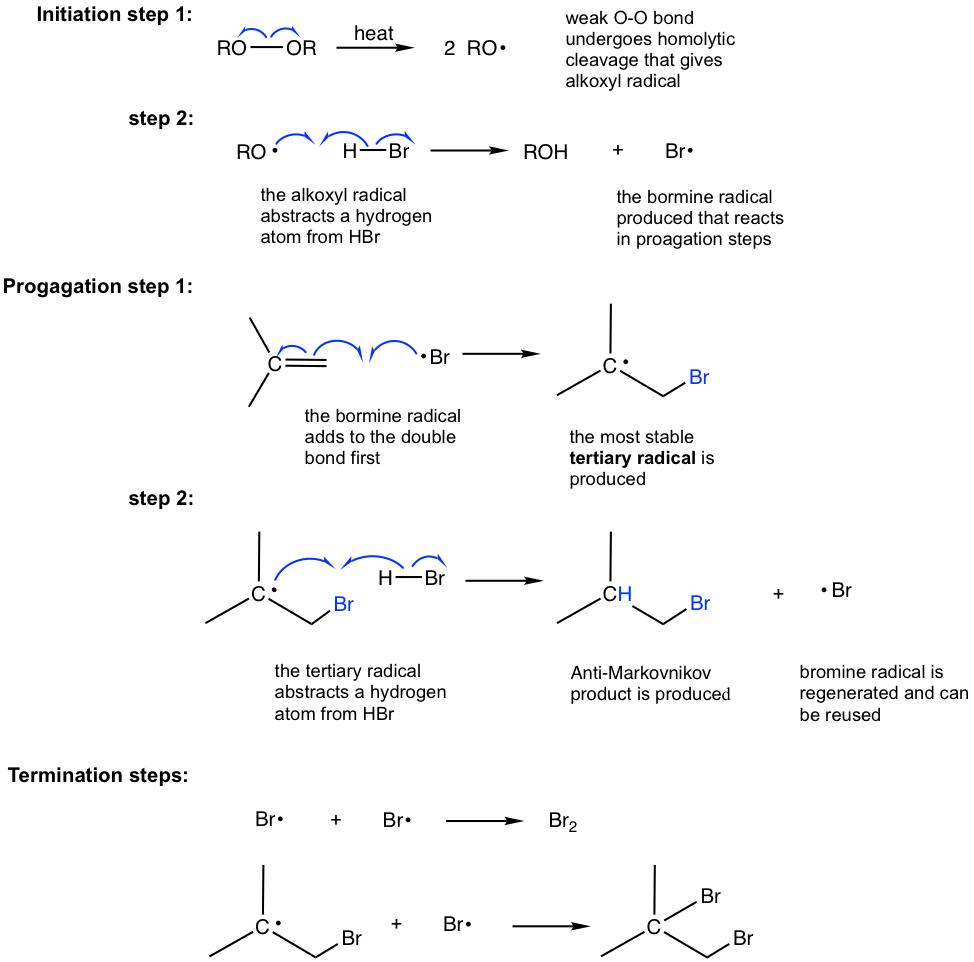
10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I

Electrophilic Addition Of Hydrogen Halides To Alkenes Youtube
Comments
Post a Comment